Grade level: 6-12
Length: 5 minutes
Next Generation Science Standards: HS-PS1.B, HS-ETS1.A
Video Synopsis
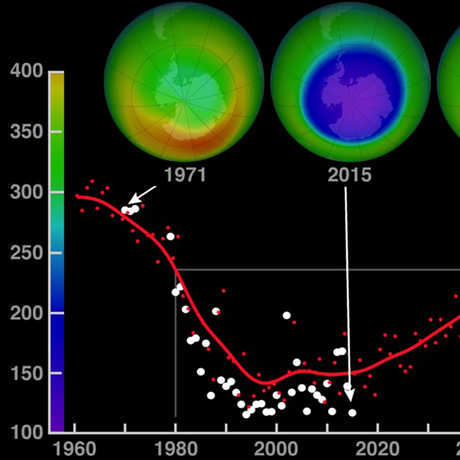
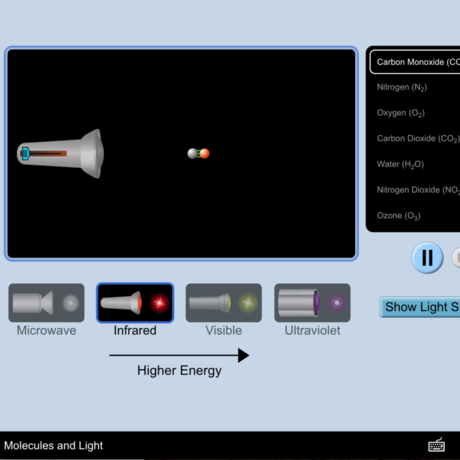
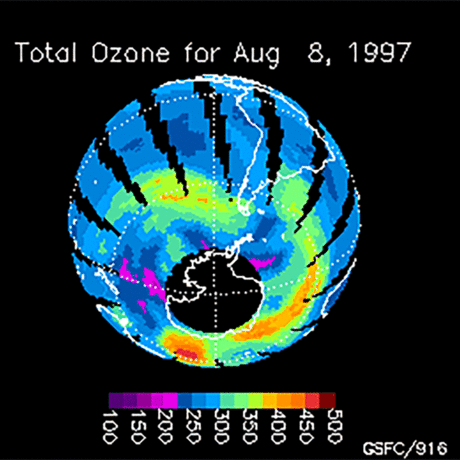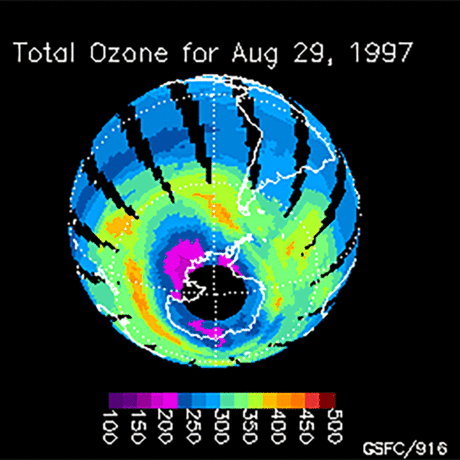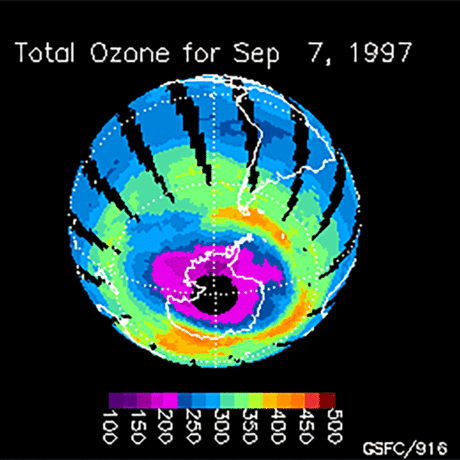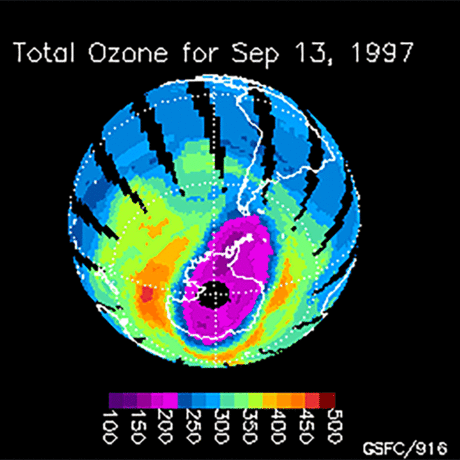
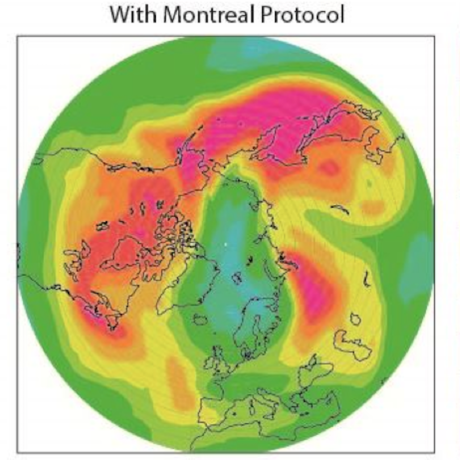
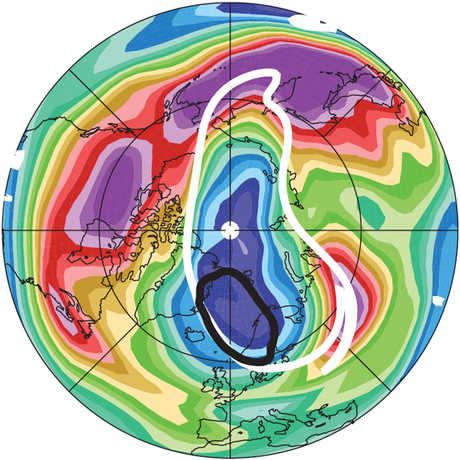
Ozone (O3) is an important gas in Earth's atmosphere that is a pollutant in the troposphere, where we live, but is a protector in the stratosphere over 10 km up. Stratospheric ozone protects us from much of the Sun's harmful ultraviolet radiation by interacting with UV rays in a series of chemical reactions that are constantly breaking ozone apart and reforming it. While this system of reactions is naturally in balance, manmade gases called chlorofluorocarbons (CFCs) that were used in refrigerators, air conditioners, and aerosol sprays act as catalysts in the destruction of ozone. In 1987, the Montreal Protocol banned the use of CFCs after is was discovered that the ozone layer over Antarctica had thinned to dangerous levels. Since 1993, CFC levels in the atmosphere have decreased and the ozone layer is making a comeback.
This video was produced in collaboration with the San Francisco Unified School District as a part of their NGSS-aligned high school chemistry curriculum.